Multiple Choice
Identify the missing particle in the following nuclear equation: 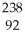 U → ? +
U → ? + He + 2
He + 2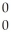 Γ
Γ
A) 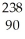 Th
Th
B) 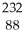 Ra
Ra
C) 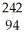 Pu
Pu
D) 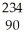 Th
Th
E) 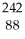 Ra
Ra
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: Above what atomic number are there no
Q15: Nuclides below the valley of stability can
Q16: If a sample initially contains 1.35 mg
Q17: Describe what changes occur during gamma ray
Q18: Describe what changes occur during alpha decay.<br>A)The
Q20: Identify the missing particle in the following
Q22: Determine the identity of the daughter nuclide
Q23: Describe what changes occur during beta decay.<br>A)The
Q24: Identify the missing particle in the following
Q84: Fluorine-18 undergoes positron emission with a half-life