Multiple Choice
Substance A has a density of 3 g/cm3 and substance B has a density of 4 g/cm3.In order to obtain equal masses of these two substances,what must be the ratio of the volume of A to the volume of B?
A) 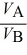 =
=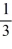
B) 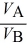 =
=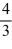
C) 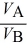 =
=
D) 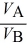 =
=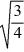
E) 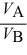 =
=
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q42: A 4.7-kg solid sphere,made of metal whose
Q43: An air bubble underwater has the same
Q44: The density of material at the center
Q45: What force does the water exert (in
Q46: Water flows out of a large reservoir
Q48: Ideal incompressible fluid flows through a 4.0-cm-diameter
Q50: The water pressure to an apartment is
Q51: A 12,000-N car is raised using a
Q52: A crane lifts a steel submarine of
Q525: Water flows through a pipe. The diameter