Multiple Choice
If you add 700 kJ of heat to 700 g of water originally at 70.0°C,how much water is left in the container? The latent heat of vaporization of water is 22.6 × 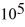
J/kg,and its specific heat capacity is 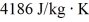
.
A) 429 g
B) 258 g
C) 340 g
D) 600 g
E) none
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: A metal has a latent heat of
Q2: A wire of diameter 0.20 mm stretches
Q3: The molecular weight of nitrogen,N<sub>2</sub>,is 28 g/mol.What
Q5: An oxygen molecule,O<sub>2</sub>,falls in a vacuum.From what
Q6: A 20.0-L pressure vessel holds 2.00 mol
Q7: A sample of an ideal gas is
Q8: How much heat must be removed from
Q9: A 5.3 L flask of ideal neon
Q10: A piano wire has a radius of
Q11: A 24.0-L tank contains ideal helium gas