Multiple Choice
How much heat must be added to a 8.0-kg block of ice at -8°C to change it to water at 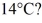
The specific heat of ice is 2050 J/kg ∙ °C,the specific heat of water is 4186 J/kg ∙ °C,the latent heat of fusion of ice is 334,000 J/kg,and 1 cal = 4.186 J.
A) 780 kcal
B) 140 kcal
C) 180 kcal
D) 810 kcal
E) 730 kcal
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q56: A 1200-kg car is being raised with
Q57: If the temperature of a gas is
Q58: A large 75-kg lighting fixture can be
Q59: A certain automobile tire has a volume
Q60: Two wires are made out of the
Q62: A solid steel column is 4.0 m
Q63: A .20-kg ice cube at 0.0°C has
Q64: A 2,294-kg sample of water at 0°
Q65: Oxygen molecules are 16 times more massive
Q66: A gas-filled vertical cylinder,closed at the bottom