Multiple Choice
A substance has a melting point of 20°C and a heat of fusion of 3.4 × 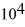
J/kg.The boiling point is 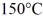
And the heat of vaporization is 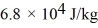
At a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid) ,1000 J/kg ∙ K (liquid) ,and 400 J/kg ∙ K (gaseous) .How much heat is required to raise the temperature of 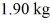
Of this substance from 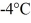
To 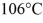
At a pressure of one atmosphere?
A) 260 kJ
B) 190 kJ
C) 230 kJ
D) 92 kJ
E) 320 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q47: An ideal gas occupies 6.00 × 10<sup>2</sup>
Q48: A sealed container holds 0.020 moles of
Q49: A 400-g block of iron at 400°C
Q50: What is the average translational kinetic energy
Q51: A vertical 30-cm steel rod,1.0 cm in
Q53: A steel wire,3.2 m long,has a diameter
Q54: An 920-g piece of iron at 100°C
Q55: A 25-kg piece of equipment can be
Q56: A 1200-kg car is being raised with
Q57: If the temperature of a gas is