Multiple Choice
At what temperature would the root mean square speed of oxygen molecules,O2,be 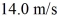
If oxygen behaves like an ideal gas? The mass of one O2 molecule is 5.312 × 10-26 kg,and the Boltzmann constant is 1.38 × 10-23 J/K.
A) 0.251 K
B) 2,090 K
C) 6,270 K
D) 1.52 × 1023 K
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: A 5.3 L flask of ideal neon
Q10: A piano wire has a radius of
Q11: A 24.0-L tank contains ideal helium gas
Q12: A car starts out when the air
Q13: What is the average translational kinetic energy
Q15: When a 125-kg piece of equipment is
Q16: A refrigerator has an interior volume of
Q17: A 35-g block of ice at -14°C
Q18: Two experimental runs are performed to determine
Q19: A 45.0-kg sample of ice is at