Multiple Choice
A rigid container is filled with 4.0 mol of air with CV = 2.5R.How much does the internal (thermal) energy of the air change if its temperature rises from 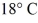
To 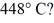
(R = 8.31 J/mol ∙ K)
A) 35,800 J
B) 430 J
C) 3,580 J
D) 8,940 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: An ideal Carnot air conditioner operates between
Q5: During an isothermal process,5.0 J of heat
Q6: When a gas expands adiabatically,<br>A)the internal (thermal)energy
Q7: During each cycle of operation,a refrigerator absorbs
Q8: A heat engine having the maximum possible
Q10: A 10-L flask and a 1-L flask
Q11: During an isochoric process,the internal (thermal)energy of
Q12: An expansion process on an ideal diatomic
Q13: The temperature of an ideal gas in
Q14: Two ideal Carnot heat engines have the