Multiple Choice
An ideal gas undergoes the process a→b→c→a shown in the pV diagram.In this figure,Pa = Pc = 3.60 × 105 Pa,Vb = Vc = 68.00 L,Va = 35 L,and Pb = 5.60 × 105 Pa.How much work is done by the system in this process? 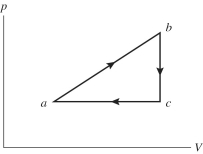
A) 2300 J
B) 3300 J
C) 2800 J
D) 3800 J
E) 3000 J
Correct Answer:

Verified
Correct Answer:
Verified
Q64: How much heat is required to raise
Q65: Suppose that the Department of Energy develops
Q66: For an ideal gas,<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="For
Q67: A heat engine receives 7000 J of
Q68: An ideal Carnot engine operates between a
Q70: In an adiabatic compression,200 J of work
Q71: Which of the following is a false
Q72: How much heat is required to increase
Q73: An ideal Carnot engine operating between a
Q74: The figure shows a pV diagram for