Multiple Choice
What is the energy (in eV) of an optical photon of frequency 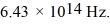
(h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A) 2.66 eV
B) 1.62 eV
C) 1.94 eV
D) 3.27 eV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q56: If the de Broglie wavelength of an
Q57: What is the wavelength of the matter
Q58: An electron is accelerated from rest through
Q59: When the surface of a metal is
Q60: How much energy is carried by a
Q62: The lattice spacing of the principal Bragg
Q63: A laser pulse of duration 25 ms
Q65: When light of wavelength 350 nm is
Q66: What is the wavelength of a 6.32-eV
Q124: Two identical metal bars are heated up