Multiple Choice
One of the emission lines described by the original version of Balmer's formula has wavelength 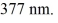
What is the value of n in Balmer's formula that gives this emission line?
A) 11
B) 12
C) 13
D) 14
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: How many electrons will fit into a
Q24: The longest wavelength of a photon that
Q25: If n = 5,which one of the
Q26: The wavelength of a ruby laser is
Q27: Given that the binding energy of the
Q29: How many values can the magnetic quantum
Q30: To which of the following values of
Q32: A hydrogen atom is in the 6h
Q33: In a hydrogen atom,an electron with n
Q52: For the ground state of the hydrogen