Multiple Choice
A hydrogen atom makes a downward transition from the 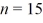
State to the 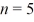
State.Find the wavelength of the emitted photon.(c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J,h = 6.626 × 10-34 J ∙ s)
A) 2.56 μm
B) 1.54 μm
C) 2.05 μm
D) 3.07 μm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q35: How many unique quantum states correspond to
Q36: How many 2d electron states can an
Q37: The Lyman series is formed by electron
Q38: What is the shortest wavelength of a
Q39: What is the shortest wavelength in the
Q41: What is the shortest wavelength of the
Q42: What is the longest wavelength in the
Q43: What value of n corresponds to a
Q44: What is the wavelength in the Balmer
Q45: Given that the speed of an electron