Multiple Choice
An atom has completely filled inner shells and a single valence electron in an excited p state.The filled inner shells have an orbital momentum equal to zero.What is the magnitude of the orbital angular momentum of the atom?
A) 1.0 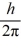
B) 1.2 
C) 1.4 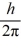
D) 1.7 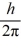
E) 2.0 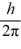
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q74: If a hydrogen atom originally in a
Q75: The orbital angular momentum quantum number ℓ
Q76: What value of n corresponds to a
Q77: Hydrogen atoms can emit four spectral lines
Q78: How many values of the magnetic quantum
Q80: If ℓ = 4,which one of the
Q81: In a transition from one vibrational state
Q82: How many electrons can be found with
Q83: How many values of the magnetic quantum
Q84: In a hydrogen atom,a given electron has