Multiple Choice
Bismuth 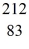
Bi is known to be radioactive.The stability of 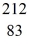
Bi with respect to alpha,beta-plus and beta-minus decay is to be determined.The following atomic masses are known: 
He: 4.002603 u 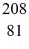
Tl: 207.981998 u 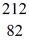
Pb: 211.991871 u 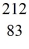
Bi: 211.991255 u 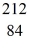
Po: 211.988842 u
The 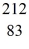
Bi nucleus is
A) subject to alpha decay only.
B) subject to beta-plus decay only.
C) subject to beta-minus decay only.
D) subject to alpha or beta-plus decay,but not beta-minus decay.
E) subject to alpha or beta-minus decay,but not beta-plus decay.
Correct Answer:

Verified
Correct Answer:
Verified
Q76: Scandium <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="Scandium Sc
Q77: How many quarks are in a tritium
Q78: In beta-minus decay<br>A)a proton is emitted.<br>B)a neutron
Q79: A sample containing 4.0 × 10<sup>18</sup> atoms
Q80: An excited <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="An excited
Q82: A 70-kg researcher absorbs 4.5 × <img
Q83: The stability of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="The stability
Q84: A nuclear explosion results in a mass
Q85: Elementary particles that experience the weak nuclear
Q86: A certain substance has a half-life of