Multiple Choice
What mass of 14C,with a half-life of 5730 y,do you need to provide a decay rate of 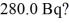
The mass of 14C is 14.00 u,and 1 u = 1.6605 × 10-27 kg.
A) 1.70 × 10-12 kg
B) 5.38 × 10-19 kg
C) 3.84 × 10-20 kg
D) 8.68 × 10-13 kg
Correct Answer:

Verified
Correct Answer:
Verified
Q84: A nuclear explosion results in a mass
Q85: Elementary particles that experience the weak nuclear
Q86: A certain substance has a half-life of
Q87: What is true of an element of
Q88: The nuclei of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="The nuclei
Q90: The radioactivity due to carbon-14 measured in
Q91: The symbol for a certain isotope of
Q92: An archaeologist finds the <sup>14</sup>C in a
Q93: Radium-226 decays into radon-222 plus an alpha
Q94: The maximum permissible workday dose for occupational