Multiple Choice
Consider the nuclear reaction: 7Li + 1p → 4He + 4He.The known atomic masses are
7Li: 7.016005 u 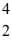
He: 4.002603 u
1p: 1.007825 u
What is the Q-value (or reaction energy) of this reaction? (1 u = 931.5 MeV/c2)
A) 13.57 MeV
B) 15.37 MeV
C) 17.35 MeV
D) 17.53 MeV
Correct Answer:

Verified
Correct Answer:
Verified
Q62: An electron and a positron,both essentially at
Q63: The isotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="The isotope
Q64: The neutral helium atom, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="The
Q65: The atomic number of an atom is
Q66: In the nuclear reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="In
Q68: A beryllium-8 nucleus at rest undergoes double
Q69: A radioactive source emits 2.4-MeV neutrons at
Q70: Consider the nuclear reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="Consider
Q71: The following masses are known: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg"
Q72: A thallium source with a half-life of