Multiple Choice
In this electrochemical cell,the reduction half reaction is 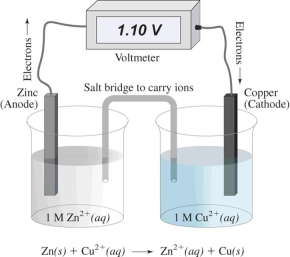
A) Cu2+(aq) + 2 e¯ → Cu(s)
B) Zn(s) → Zn2+(aq) + 2 e¯
C) Zn(s) → Cu(s)
D) Cu2+(aq) → Zn2+(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: Which is not a valid argument supporting
Q11: Which is not a current or planned
Q14: Which is not a component of a
Q16: A fuel cell does not "run down"
Q17: What is the primary determinant of the
Q18: What is the purpose of the hot
Q19: Why can the lead-acid batteries used in
Q20: As fuel cells become more widely accepted
Q38: To date,solar radiation is not a practical
Q49: Sunlight (solar radiation) may be turned directly