Multiple Choice
How does the reaction of hydrogen and oxygen to produce energy in a fuel cell differ from their interaction during the direct combustion of hydrogen and oxygen? 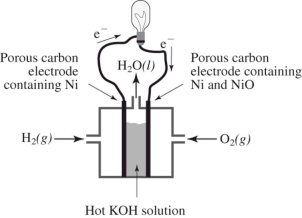
I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only water.
II.Much less heat energy is produced in a fuel cell than via direct combustion of hydrogen and oxygen.
III.In the fuel cell,there is an oxidation-reduction reaction between hydrogen and oxygen.In the direct combustion of hydrogen and oxygen,there is no such reaction.
IV.It is much easier to control the hydrogen and oxygen during direct combustion than during their reaction in a fuel cell.
A) I and II only
B) I,II and III only
C) I,II,III and IV
D) I and III only
Correct Answer:

Verified
Correct Answer:
Verified
Q14: Which increases the efficiency of a photovoltaic
Q42: Semiconductors,such as the element silicon,may be used
Q48: In the lead-acid (storage)battery, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2349/.jpg" alt="In
Q49: The current through a wire is most
Q50: In an electrochemical cell,the anode is<br>A)the electrode
Q51: The Prius,a hybrid car produced by Toyota,uses
Q52: Batteries must be used in addition to
Q55: A candle may be considered a more
Q57: At present,it will be difficult and perhaps
Q58: Which has not been suggested as a