Multiple Choice
Object 1 has three times the specific heat capacity and four times the mass of Object 2. The two objects are given the same amount of heat. If the temperature of Object 1 changes by an amount ΔT, the change in temperature of Object 2 will be
A) ΔT.
B) 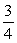 ΔT.
ΔT.
C) 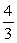 ΔT.
ΔT.
D) 6ΔT.
E) 12ΔT.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: What is the net power that a
Q15: Gas in a constant-volume gas thermometer registers
Q16: Heat is energy that is transferred between
Q17: At what rate is the human body
Q18: When you sit by a campfire, by
Q20: Does it make sense to say that
Q20: The coefficient of linear expansion of copper
Q21: The type of heat transfer that occurs
Q22: At what temperature are the numerical readings
Q24: What is the ratio of the pressure