Multiple Choice
Object 1 has three times the specific heat capacity and four times the mass of Object 2. The two objects are heated from the same initial temperature, T0, to the same final temperature Tf. You determine that the amount of heat gained by Object 1 is Q. The amount of heat absorbed by Object 2 will be
A) 12Q.
B) 6Q.
C) 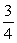 Q.
Q.
D) 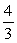 Q.
Q.
E) 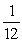 Q.
Q.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: 0.45 kg of a metal at 90.°C
Q8: The coefficient of linear expansion of copper
Q9: At room temperature, a typical person loses
Q10: FIGURE 16-3 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6213/.jpg" alt="FIGURE 16-3
Q11: The coefficient of linear expansion of copper
Q14: What is the net power that a
Q15: Gas in a constant-volume gas thermometer registers
Q16: Heat is energy that is transferred between
Q17: At what rate is the human body
Q73: The weather outside is frightful.The temperature is