Multiple Choice
A sample of an ideal gas is heated and its Kelvin temperature doubles. What happens to the average speed of the molecules in the sample?
A) It does not change.
B) It halves.
C) It doubles.
D) It is 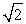 times as large.
times as large.
E) It is 1/ 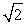 times smaller.
times smaller.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q39: A 1.00-kg piece of iron at 655°C
Q40: How much heat must be removed from
Q41: When a mixture of gases is placed
Q42: Using K = 3/2 kT, what is
Q43: Opposing shearing forces of 3000 N are
Q45: You have had something simmering in a
Q46: A container of negligible heat capacity has
Q47: A 600-g piece of iron at 100°C
Q48: Steam burns can be much more severe
Q49: When a spray can is being used,