Multiple Choice
Two containers of equal volume each hold samples of the same ideal gas. Container A has twice as many molecules as container B. If the gas pressure is the same in the two containers, the correct statement regarding the absolute temperatures TA and TB in containers A and B, respectively, is
A) TA = TB .
B) TA = 2TB .
C) TA = 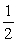 TB .
TB .
D) TA = 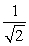 TB .
TB .
E) TA = 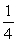 TB .
TB .
Correct Answer:

Verified
Correct Answer:
Verified
Q53: When a solid melts,<br>A) the temperature of
Q76: The heat required to change a substance
Q77: When we say that a material sample
Q79: Two moles of gas are at STP.
Q80: Which of the following best explains why
Q82: A laboratory vacuum pump can reduce the
Q83: The melting point of aluminum is 660°C,
Q84: The internal energy of an ideal gas
Q85: A vertical cylindrical cylinder, closed at the
Q86: What is the rms speed of a