Multiple Choice
Uranium-238 decays into thorium-234 plus an alpha particle. How much energy is released in this process? 1 u = 931.494 MeV/c2. The relevant mass values are: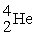 , 4.002603 u;
, 4.002603 u; 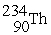 , 234.043583 u;
, 234.043583 u; 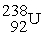 , 238.050786 u.
, 238.050786 u.
A) 4.28 MeV
B) 3.76 MeV
C) 3.18 MeV
D) 2.89 MeV
E) 5.05 MeV
Correct Answer:

Verified
Correct Answer:
Verified
Q4: An old bone sample contains 321. grams
Q5: The competition between the electrostatic force between
Q7: Fermium-253 has a half-life of 3.00 days.
Q8: Elementary particles that experience the weak nuclear
Q11: Composite particles that are composed of two
Q12: When a neutron collides with a uranium-235
Q13: Composite particles that are composed of three
Q13: The decay constant of radon is 0.181
Q14: What is the nuclear radius of <sup>90</sup>Sr?<br>A)
Q19: What combination of quarks produces a proton