Multiple Choice
If the heat of fusion for water is 80.cal/g,how many calories are needed to melt 45.0 g of ice at 0 °C?
A) 3.6 cal
B) 3.6 × 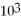 cal
cal
C) 1.8 cal
D) 80.cal
E) 0.56 cal
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: Absolute zero is _.<br>A)the freezing point of
Q22: Which of the following is an example
Q23: Which of the following is an element?<br>A)tin<br>B)water<br>C)salt<br>D)sugar<br>E)iced
Q32: How many calories are required to raise
Q43: A serving of fish contains 50 g
Q53: Match the state(s)of matter with each of
Q63: On a heating curve a plateau corresponds
Q76: In which of the following would the
Q89: When a liquid boils,the process by which
Q93: Raising the temperature of 10.0 g of