Multiple Choice
The carbon tetrachloride molecule,CC 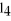 ,is ________.
,is ________.
A) a polar molecule with polar bonds
B) a nonpolar molecule with polar bonds
C) a nonpolar molecule with nonpolar bonds
D) a polar molecule with nonpolar bonds
E) a polar molecule with ionic bonds
Correct Answer:

Verified
Correct Answer:
Verified
Q20: For halogens,the group number is the same
Q58: The ability of an atom to attract
Q89: Match the correct name of the polyatomic
Q91: The name of the compound CO<sub>2 </sub>is
Q93: The strongest attractive forces between molecules of
Q95: When Al<sup>3+</sup> and SO<sub>4</sub><sup>2-</sup> combine,the formula of
Q95: An ionic compound _.<br>A)has a net positive
Q96: The ion of aluminum is _.<br>A) <img
Q97: Methane,(CH<sub>4</sub>),is a polar molecule.
Q98: The name of the compound K<sub>3</sub>N is