Multiple Choice
Given the following equation,what is the correct form of the conversion factor needed to convert the number of moles of 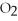 to the number of moles of
to the number of moles of 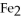
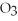 produced?
produced?
4Fe + 3 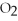 → 2
→ 2 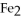
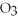
A) 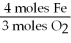
B) 
C) 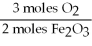
D) 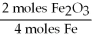
E) 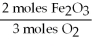
Correct Answer:

Verified
Correct Answer:
Verified
Q12: The molar mass of silver is 47
Q41: In an oxidation-reduction reaction,the substance reduced always
Q88: What is the mass,in grams,of 1.00 mole
Q94: For the following question(s),consider the following equation.<br>2Mg
Q95: For the following question(s),consider the following balanced
Q97: How many moles of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="How
Q100: The following is a double replacement reaction.<br>2
Q102: The following reaction is an example of
Q104: How many moles of carbon atoms are
Q106: In a _ reaction,two or more elements