Multiple Choice
For the following question(s) ,consider the following equation.
2Mg + 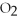 → 2MgO
→ 2MgO
-The number of moles of MgO produced when 0.20 mole of 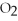 reacts completely is ________.
reacts completely is ________.
A) 0.10 mole
B) 0.20 mole
C) 0.40 mole
D) 0.60 mole
E) 0.80 mole
Correct Answer:

Verified
Correct Answer:
Verified
Q53: The reaction of carbon with oxygen to
Q62: The molar mass of calcium hydroxide, <img
Q63: In the following reaction,when 100.g of Br<sub>2</sub>
Q64: The molar mass of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="The
Q66: Barium chloride and sodium sulfate react according
Q68: Which of the following is an oxidation-reduction
Q68: The molar mass of magnesium is 24.31
Q69: If the reaction shown below is exothermic,the
Q70: For the following question(s),consider the following balanced
Q71: Barium chloride and sodium sulfate react according