Multiple Choice
For the following question(s) ,consider the following equation.
2Mg + 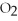 → 2MgO
→ 2MgO
-How many grams of magnesium are needed to react with 16 g of 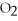 ?
?
A) 0.50 g
B) 1.0 g
C) 12 g
D) 24 g
E) 48 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: In the following reaction,what is the correct
Q31: What is the classification for this unbalanced
Q32: The mass of one mole of water
Q33: When 25.0 g of KCl <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg"
Q34: What is the molar mass of sodium
Q36: How many grams of glucose ( <img
Q38: Pentane ( C<sub>5</sub>H<sub>12</sub> )reacts with oxygen
Q59: Barium chloride and sodium sulfate react according
Q82: Barium chloride and sodium sulfate react according
Q102: 0.100 mole of lithium has a mass