Multiple Choice
For the following question(s) ,consider the following balanced equation. 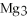
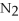 + 6
+ 6 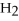 O → 3
O → 3 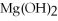 + 2
+ 2 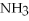
-How many grams of NO are required to produce 145 g of 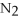 in the following reaction?
in the following reaction?
4 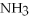 + 6NO → 5
+ 6NO → 5 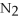 + 6
+ 6 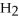 O
O
A) 186 g
B) 155 g
C) 125 g
D) 129 g
E) 145 g
Correct Answer:

Verified
Correct Answer:
Verified
Q11: Pentane ( C<sub>5</sub>H<sub>12</sub> )reacts with oxygen
Q19: One mole of particles of any substance
Q20: What is the coefficient of hydrogen, <img
Q21: 0.400 mole of sucrose, C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>,contains _ C
Q22: What is oxidized and what is reduced
Q23: What is the classification for this reaction?
Q27: Avogadro's number is the number of _.<br>A)particles
Q29: How many moles of water, H<sub>2</sub>O,are present
Q44: The molar mass of chlorine gas is
Q46: Pentane ( C<sub>5</sub>H<sub>12</sub> )reacts with oxygen