Multiple Choice
For the following question(s) ,consider the following balanced equation. 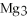
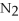 + 6
+ 6 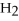 O → 3
O → 3 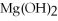 + 2
+ 2 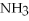
-How many grams of 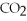 are produced from 125 g of
are produced from 125 g of 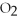 and excess
and excess 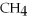 ?
?
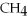 + 2
+ 2 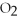 →
→ 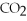 + 2
+ 2 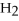 O
O
A) 125 g
B) 62.5 g
C) 172 g
D) 85.9 g
E) 250.g
Correct Answer:

Verified
Correct Answer:
Verified
Q2: In any balanced chemical equation,the number of
Q4: What is the molar mass of sucrose
Q6: For the following reaction,what is the correct
Q8: Which reactant is oxidized in the following
Q9: In this reaction,what is the correct coefficient
Q11: The molar mass of potassium is _.<br>A)19.00
Q39: Any reaction that absorbs 150 kcal of
Q47: For the following question(s),consider the following equation.<br>2Mg
Q62: Answer the question(s)below about the following reaction.<br>2
Q97: A chemical equation is balanced when _.<br>A)the