Multiple Choice
Answer the question(s) below about the following reaction.
2 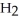
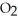 → 2
→ 2 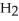 O +
O + 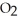
-How many grams of hydrogen peroxide are needed to produce 25.0 g of oxygen?
A) 106 g
B) 26.6 g
C) 5.88 g
D) 25.0 g
E) 53.2 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q75: Which of the following gives the balanced
Q76: The mass of 0.352 mole of sodium
Q77: In the following reaction,when 100.g of Br2
Q78: One mole of neon atoms has a
Q79: What type of reaction is the following?<br>Zn
Q81: The following is a decomposition reaction.<br>2KCl <img
Q82: Barium chloride and sodium sulfate react according
Q83: What coefficient is placed in front of
Q84: How many moles of K<sub>2</sub>SO<sub>4</sub> are
Q85: For the following question(s),consider the following equation.<br>2Mg