Matching
Identify the following as acids, bases, or neutral solutions.
Premises:
has a sour taste
pH =9.0
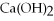
has a pH = 4.5
[ ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bc_91e5_d575360158c3_TB6077_11.jpg)
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bd_91e5_0d5857e563bf_TB6077_11.jpg) ] = 1.0 ×
] = 1.0 × ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9be_91e5_9d908279e706_TB6077_11.jpg) M
M
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bc_91e5_d575360158c3_TB6077_11.jpg)
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bd_91e5_0d5857e563bf_TB6077_11.jpg) ] = 1.0 ×
] = 1.0 × ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9be_91e5_9d908279e706_TB6077_11.jpg) M
Mcontains more hydronium ions than hydroxide ions
[ ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_9b97_91e5_6d910dcac0ce_TB6077_11.jpg)
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a8_91e5_d1f18392ff63_TB6077_11.jpg) ] = 3.4 ×
] = 3.4 × ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a9_91e5_2325e66902c8_TB6077_11.jpg) M
M
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_9b97_91e5_6d910dcac0ce_TB6077_11.jpg)
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a8_91e5_d1f18392ff63_TB6077_11.jpg) ] = 3.4 ×
] = 3.4 × ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a9_91e5_2325e66902c8_TB6077_11.jpg) M
M[ ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2aa_91e5_2302ac22655c_TB6077_11.jpg) ]= 2.8 ×
]= 2.8 × ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2ab_91e5_75c18cb93847_TB6077_11.jpg) M
M
![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2aa_91e5_2302ac22655c_TB6077_11.jpg) ]= 2.8 ×
]= 2.8 × ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2ab_91e5_75c18cb93847_TB6077_11.jpg) M
M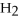 O
Oturns blue litmus paper red
Responses:
base
acid
neutral
Correct Answer:
Premises:
Responses:
has a sour taste
pH =9.0
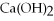
has a pH = 4.5
[ ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bc_91e5_d575360158c3_TB6077_11.jpg)
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bd_91e5_0d5857e563bf_TB6077_11.jpg) ] = 1.0 ×
] = 1.0 × ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9be_91e5_9d908279e706_TB6077_11.jpg) M
M
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bc_91e5_d575360158c3_TB6077_11.jpg)
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bd_91e5_0d5857e563bf_TB6077_11.jpg) ] = 1.0 ×
] = 1.0 × ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9be_91e5_9d908279e706_TB6077_11.jpg) M
Mcontains more hydronium ions than hydroxide ions
[ ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_9b97_91e5_6d910dcac0ce_TB6077_11.jpg)
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a8_91e5_d1f18392ff63_TB6077_11.jpg) ] = 3.4 ×
] = 3.4 × ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a9_91e5_2325e66902c8_TB6077_11.jpg) M
M
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_9b97_91e5_6d910dcac0ce_TB6077_11.jpg)
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a8_91e5_d1f18392ff63_TB6077_11.jpg) ] = 3.4 ×
] = 3.4 × ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a9_91e5_2325e66902c8_TB6077_11.jpg) M
M[ ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2aa_91e5_2302ac22655c_TB6077_11.jpg) ]= 2.8 ×
]= 2.8 × ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2ab_91e5_75c18cb93847_TB6077_11.jpg) M
M
![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2aa_91e5_2302ac22655c_TB6077_11.jpg) ]= 2.8 ×
]= 2.8 × ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2ab_91e5_75c18cb93847_TB6077_11.jpg) M
M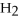 O
Oturns blue litmus paper red
Premises:
has a sour taste
pH =9.0
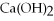
has a pH = 4.5
[ ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bc_91e5_d575360158c3_TB6077_11.jpg)
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bd_91e5_0d5857e563bf_TB6077_11.jpg) ] = 1.0 ×
] = 1.0 × ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9be_91e5_9d908279e706_TB6077_11.jpg) M
M
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bc_91e5_d575360158c3_TB6077_11.jpg)
![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9bd_91e5_0d5857e563bf_TB6077_11.jpg) ] = 1.0 ×
] = 1.0 × ![[ ] = 1.0 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_e9be_91e5_9d908279e706_TB6077_11.jpg) M
Mcontains more hydronium ions than hydroxide ions
[ ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_9b97_91e5_6d910dcac0ce_TB6077_11.jpg)
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a8_91e5_d1f18392ff63_TB6077_11.jpg) ] = 3.4 ×
] = 3.4 × ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a9_91e5_2325e66902c8_TB6077_11.jpg) M
M
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_9b97_91e5_6d910dcac0ce_TB6077_11.jpg)
![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a8_91e5_d1f18392ff63_TB6077_11.jpg) ] = 3.4 ×
] = 3.4 × ![[ ] = 3.4 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2a9_91e5_2325e66902c8_TB6077_11.jpg) M
M[ ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2aa_91e5_2302ac22655c_TB6077_11.jpg) ]= 2.8 ×
]= 2.8 × ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2ab_91e5_75c18cb93847_TB6077_11.jpg) M
M
![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2aa_91e5_2302ac22655c_TB6077_11.jpg) ]= 2.8 ×
]= 2.8 × ![[ ]= 2.8 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de55_c2ab_91e5_75c18cb93847_TB6077_11.jpg) M
M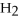 O
Oturns blue litmus paper red
Responses:
Related Questions
Q10: Alkalosis is the blood condition in which
Q14: An aqueous solution with <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="An
Q16: A solution with [ <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="A
Q17: Which of the following statements correctly describes
Q18: In any aqueous solution, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="In
Q21: Which of the following is a buffer
Q27: In a neutralization reaction _.<br>A)two acids react
Q39: For many reactions of acids with bases,the
Q44: When a system is at equilibrium,all the
Q45: The function of a buffer is to