Multiple Choice
According to the Arrhenius concept,if 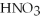 were dissolved in water,it would act as ________.
were dissolved in water,it would act as ________.
A) a base
B) an acid
C) a source of hydroxide ions
D) a source of H- ions
E) a proton acceptor
Correct Answer:

Verified
Correct Answer:
Verified
Q7: In the following solutions,is the [OH-] greater
Q9: In any aqueous solution, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="In
Q10: Alkalosis is the blood condition in which
Q12: When hyperventilation (rapid breathing)causes a patient to
Q13: The [ H<sub>3</sub>O<sup>+</sup> ] of a solution
Q14: An aqueous solution with <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="An
Q16: A solution with [ <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="A
Q17: What is the name of the medical
Q34: An acid and base react to form
Q58: When a reaction is at equilibrium,_.<br>A)all reaction