Multiple Choice
According to LeChâtelier's principle,predict whether adding 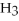
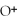 causes the system to shift in the direction of reactants,products,or no change.
causes the system to shift in the direction of reactants,products,or no change.
N 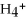 (aq) + H2O(l) ⇌ N
(aq) + H2O(l) ⇌ N 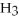 (aq) +
(aq) + 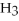
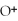 (aq)
(aq)
A) reactants
B) products
C) no change
Correct Answer:

Verified
Correct Answer:
Verified
Q21: Which of the following is a buffer
Q22: A buffer is a solution that tends
Q25: For <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="For ,the
Q26: A system is at equilibrium when the
Q27: In a neutralization reaction _.<br>A)two acids react
Q27: Identify each of the following compounds as
Q28: The [ H<sub>3</sub>O<sup>+</sup> ] of a solution
Q31: The neutralization reaction between <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="The
Q39: For many reactions of acids with bases,the
Q45: The function of a buffer is to