Multiple Choice
According to LeChâtelier's principle,predict whether adding H2O causes the system to shift in the direction of reactants,products,or no change.
N 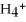 (aq) +
(aq) + 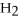 O(l) ⇌ N
O(l) ⇌ N 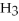 (aq) +
(aq) + 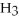
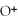 (aq)
(aq)
A) reactants
B) products
C) no change
Correct Answer:

Verified
Correct Answer:
Verified
Q5: A solution with a pH greater than
Q22: A buffer is a solution that tends
Q26: A system is at equilibrium when the
Q28: The [ H<sub>3</sub>O<sup>+</sup> ] of a solution
Q31: The neutralization reaction between <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="The
Q34: The conjugate base of H<sub>2</sub>O is _.<br>A)
Q36: Which of the following is a neutralization
Q37: Strong acids react with Zn metal to
Q38: Which one of the following is characteristic
Q63: Ammonium hydroxide is a weak base because