Multiple Choice
Consider the following specific heats of metals. 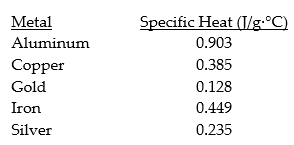
If the same amount of heat is added to 50.0 g samples of each of the metals,which are all at the same temperature,which metal will reach the highest temperature?
A) aluminum
B) copper
C) gold
D) iron
E) silver
Correct Answer:

Verified
Correct Answer:
Verified
Q46: How much heat (kJ)is absorbed by 948.0
Q47: Mixtures of miscible liquids that differ in
Q48: Which among the following statements is false?<br>A)A
Q49: How would you classify raisin bran?<br>A)pure substance-compound<br>B)mixture-heterogeneous<br>C)pure
Q50: Which of the following items is a
Q52: A 15.0 gram lead ball at 25.0°C
Q53: Which of the following is an example
Q54: The coldest temperature possible is 0 K.
Q55: Which of the following items is a
Q56: Sugar is a pure substance.