Multiple Choice
Suppose it took 108 joules of energy to raise a bar of gold from 25.0°C to 29.7°C.Given that the specific heat capacity of gold is 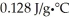 ,what is the mass (in grams) of the bar of gold?
,what is the mass (in grams) of the bar of gold?
A) 6.5 × 101 g
B) 1.8 × 102 g
C) 1.28 × 102 g
D) 1.08 × 102 g
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q8: What type of energy is associated with
Q9: Which type of energy is associated with
Q10: When you dissolve solid sugar into water,this
Q11: How many Calories are in 575.0 calories?<br>A)575,000<br>B)0.5750<br>C)137.6<br>D)2,404<br>E)none
Q12: Which of the following is a homogeneous
Q14: Which of the following statements about physical
Q15: A chemical change that will lower the
Q16: When methane is burned with oxygen the
Q17: A pure substance is:<br>A)composed of two or
Q18: In order,what is the freezing point,room temperature