Multiple Choice
Given the table of specific heat values below,what is the identity of a 10.0 g metal sample that increases by 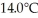 when
when 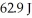 of energy is absorbed?
of energy is absorbed? 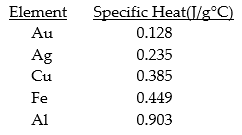
A) Fe
B) Al
C) Au
D) Ag
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What is the value of 783 K
Q2: When methane is burned with oxygen,the products
Q4: Which state of matter has atomic spacing
Q5: In a chemical reaction,the substances present after
Q6: An energy diagram that shows the reactants
Q7: What is the final temperature of 25.0
Q8: What type of energy is associated with
Q9: Which type of energy is associated with
Q10: When you dissolve solid sugar into water,this
Q11: How many Calories are in 575.0 calories?<br>A)575,000<br>B)0.5750<br>C)137.6<br>D)2,404<br>E)none