Multiple Choice
Given the table of specific heat values below,what is the identity of a 26.2 g metal sample that increases by 8.5°C when 100.0 J of energy is absorbed? 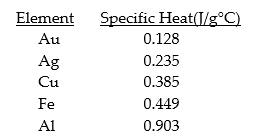
A) Fe
B) Al
C) Au
D) Ag
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q75: A moving bowling ball has kinetic energy.
Q76: The corrosion of iron is a physical
Q77: If a particular process is endothermic,the reverse
Q78: How much heat (kJ)is needed to raise
Q79: How many kilojoules are there in 95.0
Q81: Matter is defined as anything that is
Q82: What is the value of -25°C on
Q83: A solid form of matter in which
Q84: Temperatures reported in the Kelvin scale cannot
Q85: The amount of heat energy needed to