True/False
The chemical formula CuBr2 indicates that this compound is composed of 1 gram of copper and 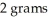 of bromine.
of bromine.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q96: A compound composed of only hydrogen and
Q97: The chemical formula CH<sub>2</sub>O can be classified
Q98: Which of the following compounds has the
Q99: The numerical value of the mole is
Q100: One mole of CO<sub>2</sub> gas contains 1
Q102: How many atoms are in 5.80 moles
Q103: C<sub>2</sub>H<sub>6</sub>O<sub>4</sub> could be an empirical formula.
Q104: How many atoms are present in 4.5
Q105: One mole of ammonium nitrite contains:<br>A)2 moles
Q106: C<sub>2</sub>H<sub>6</sub>O<sub>3</sub> could be an empirical formula.