True/False
The correct formula for calculating mass percent of X in compound XY is: 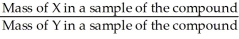 = Mass % X
= Mass % X
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: One mole of lead(II)nitrate contains six moles
Q2: How many molecules of sulfur trioxide are
Q3: A molecule that has an empirical formula
Q5: The molar mass of a compound in
Q6: Calculate the molar mass of calcium nitrate.<br>A)136.03
Q7: How many moles of Pb are in
Q8: One mole of oxygen gas has a
Q9: One mole of argon has more atoms
Q10: Which of the following is already in
Q11: The empirical formula for C<sub>6</sub>H<sub>6</sub> is C<sub>3</sub>H<sub>3</sub>.