Multiple Choice
If 3.011 ×1023 molecules have a mass of 20.04 grams,what is the molar mass of this substance?
A) 40.08 g/mol
B) 10.02 g/mol
C) 20.04 g/mol
D) 6.658 × 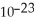 g/mol
g/mol
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q42: How many moles of fluorine are in
Q43: What is the molar mass of aluminum
Q44: C<sub>2</sub>H<sub>3</sub>O<sub>2</sub> could be an empirical formula.
Q45: What is the mass percent of hydrogen
Q46: A 42.7 gram sample of potassium nitrate
Q48: What is the mass of 1.56 ×
Q49: How many moles of bromine gas are
Q50: A chromium oxide compound contains 104.0 grams
Q51: One mole of copper atoms is 6.022
Q52: The empirical formula mass must be 25.0