Multiple Choice
If 2.01 × 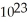 atoms of an element from Group IA of the periodic table has a mass of 7.675 grams,this element is most likely:
atoms of an element from Group IA of the periodic table has a mass of 7.675 grams,this element is most likely:
A) Li.
B) Na.
C) K.
D) Rb.
E) Cs.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q54: Two moles of cobalt atoms have a
Q55: The number 6.022 × <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6110/.jpg" alt="The
Q56: How many hydrogen atoms are in 35.0
Q57: How many moles of oxygen are in
Q58: A compound composed of only carbon and
Q60: One mole of ammonium nitrate contains:<br>A)3 moles
Q61: The mass of one mole of carbon
Q62: You have 10.0 g each of Na,C,Pb,Cu
Q63: An unknown acid has a molar mass
Q64: How many atoms are in 1.50 moles