Multiple Choice
How many atoms of lithium are in 18.7 g?
A) 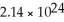
B) 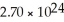
C) 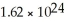
D) 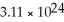
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q74: One mole of chlorine gas has a
Q75: The empirical formula mass is 18.0 and
Q76: You have 10.0 g each of Na,C,Pb,Cu
Q77: Which of the following statements about the
Q78: Calculate the molar mass of ammonium carbonate.<br>A)78.05
Q80: Bananas cost 33¢ per pound.If you spent
Q81: A 500.gram iron ore sample was determined
Q82: What is the mass (in grams)of 3.03
Q83: A 7.96 gram sample of silver reacts
Q84: One mole of nitrogen gas contains (2)×