Multiple Choice
Which is the limiting reactant in the following reaction given that you start with 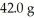 of CO2 and 99.9 g KOH?
of CO2 and 99.9 g KOH?
Reaction: CO2 + 2KOH → K2CO3 + H2O
A) K2CO3
B) H2O
C) CO2
D) KOH
E) not enough information
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: Consider the following equation: CO + 2
Q6: If 16.0 grams of aluminum oxide were
Q7: For the following reaction you have 8
Q8: How many eggs are needed to make
Q9: A chemist wishes to perform the following
Q11: When viewing a chemical equation,the limiting reactant
Q12: Suppose a computer manufacturer can build circuit
Q13: How many moles of calcium chloride are
Q14: For the following reaction you have 8
Q15: The actual yield is the same as