Multiple Choice
What is the theoretical yield in grams of CuS for the following reaction given that you start with 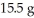 of Na2S and
of Na2S and 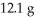 CuSO4?
CuSO4?
Reaction: Na2S + CuSO4 → Na2SO4 + CuS
A) 0.0758
B) 0.198
C) 18.93
D) 7.25
E) not enough information
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: How many moles of calcium chloride are
Q14: For the following reaction you have 8
Q15: The actual yield is the same as
Q16: A 24.0 g sample of nitrogen gas
Q17: Consider the following reaction: 2 Mg +
Q19: Given the following generic equation,2 A +
Q20: Given the balanced equation CH<sub>4</sub> + 2
Q21: What is the excess reactant for the
Q22: The limiting reactant is the reactant that
Q23: Given the balanced equation CO<sub>2</sub> + Si