Multiple Choice
Hydrochloric acid reacts with barium hydroxide according to the equation: 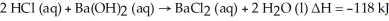 Calculate the heat (in kJ) associated with the complete reaction of 18.2 grams of HCl (aq) .
Calculate the heat (in kJ) associated with the complete reaction of 18.2 grams of HCl (aq) .
A) -58.9
B) +58.9
C) -29.5
D) -236
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q73: The percent yield can never be greater
Q74: The primary source for the rising carbon
Q75: The actual yield is the amount of
Q76: The enthalpy of reaction,△H <sub>rxn</sub>,is the amount
Q77: Before determining conversion factors,it is necessary to
Q79: What is the limiting reactant for the
Q80: How many grams of water are made
Q81: If it takes 2 cups of milk
Q82: Which ingredient is the limiting reactant if
Q83: How many grams of calcium phosphate are