True/False
The possible values for the principal quantum numbers are: 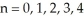 .
.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q73: What do the alkali metals all have
Q74: The ground state is when an electron
Q75: The lowest energy orbital in the quantum-mechanical
Q76: How many electrons are unpaired in the
Q77: Which statement below does NOT follow the
Q79: Which form of electromagnetic radiation has photons
Q80: Light travels through space at a speed
Q81: Which form of electromagnetic radiation has the
Q82: The energy of each Bohr orbit increases
Q83: The correct electron configuration for magnesium is: