Multiple Choice
A 250 gram sample of water at the boiling point had 45.0 kJ of heat added.How many grams of water were vaporized?
Heat of vaporization for water is 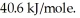
A) 1.11
B) 20.0
C) 0.902
D) 16.2
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q87: You can increase the vapor pressure of
Q88: Copper is which type of solid?<br>A)molecular solid<br>B)ionic
Q89: Rank the compounds NH<sub>3</sub>,CH<sub>4</sub>,and PH<sub>3</sub> in order
Q90: The measure of the resistance to the
Q91: Water has unusual physical properties which enable
Q93: How many kJ of heat are needed
Q94: Which state of matter has a low
Q95: Intermolecular forces determine if a substance is
Q96: Which statement is TRUE in describing what
Q97: When one mole of water vapor at