Multiple Choice
What is the value of the ion product constant for water ( Kw ) ?
A) 0.0
B) 1.0 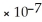
C) 1.0 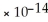
D) 1.0 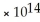
E) 1.0 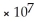
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q69: A pH of 7 is equivalent to
Q70: What is the conjugate acid of OH⁻?<br>A)
Q71: Ammonia (NH<sub>3</sub>)ionizes in water to form a
Q72: The Bronsted-Lowry definition of a base is:<br>A)a
Q73: A solution that has a pH of
Q75: In which solution is the [H<sub>3</sub>O<sup>+</sup>] less
Q76: Consider a 1.0 X 10<sup>-7</sup> M HCl
Q77: What is the concentration of H⁺ in
Q78: The pH of a 0.0001 M NaOH
Q79: In the following reaction: HCO<sub>3</sub><sup>- </sup>(aq)+ H<sub>2</sub>O