Multiple Choice
Identify the substance being oxidized in the following reaction: 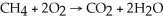 .
.
A) CH4
B) O2
C) CO2
D) H2O
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: Assign the oxidation state of each atom
Q15: Flashlight batteries are called dry cells.
Q16: For the reaction Co + Cl<sub>2</sub> →
Q17: The cathode is the electrode at which
Q18: Which of the following are typically TRUE
Q20: For the reaction Si (s)+ O<sub>2 </sub>(g)→
Q21: From the activity list included in this
Q22: Balance the redox reaction in acid solution:
Q23: Oxidation involves which of the following? <br>1.Loss
Q24: Oxidation can be defined as the gain